Your Rutherford scattering animation images are available. Rutherford scattering animation are a topic that is being searched for and liked by netizens today. You can Find and Download the Rutherford scattering animation files here. Download all royalty-free photos.
If you’re looking for rutherford scattering animation pictures information linked to the rutherford scattering animation keyword, you have come to the right site. Our website always provides you with suggestions for refferencing the highest quality video and picture content, please kindly hunt and locate more enlightening video content and images that fit your interests.
Rutherford Scattering Animation. A steady rain of alpha particles red circles comes from a source on the left. Rutherford directed the GeigerMarsden experiment in 1909 which suggested upon Rutherfords 1911 analysis that J. The data were explained by making the following assumptions. Just a little testFollow me on twitter andreabello00.
 Rutherford Scattering Experiment Ppt Video Online Download From slideplayer.com
Rutherford Scattering Experiment Ppt Video Online Download From slideplayer.com
Two of his students Hans Geiger and Ernest Marsden an undergraduate set out to measure the number of alpha particles scattered out of a collimated beam upon hitting a thin metal foil. Just a little testFollow me on twitter andreabello00. Nθ nt4r 2 zZ2K 2 e 2 4πε 0 2 1sin 4 θ2 48. Opposite the gold foil is a zinc sulfide screen that emits a flash of light when struck by an alpha particle. Rutherford Streuung - PhET Interactive Simulations. For a more quantitative approach more able students can measure the ratio of deflection angles for particles on different approach trajectories and compare this with Rutherfords scattering formula.
That was Rutherford Atomic Model and obviously discovery of NUCLEUS 6 7.
Rutherford Scattering Let us start from the one of the first steps which was done towards understanding the deepest structure of matter. The Rutherford Experiment. A cloud of negatively charged electrons surrounds this nucleus. For a more quantitative approach more able students can measure the ratio of deflection angles for particles on different approach trajectories and compare this with Rutherfords scattering formula. Atomic electrons would have negligible effect on the trajectories of the alpha particles. The tutorial simulates diffraction of alpha particles helium nuclei containing two positive charges by a thin foil made of gold metal.
 Source: slideplayer.com
Source: slideplayer.com
Rutherford Scattering Interactive Animation This animation allows you to simulate the Rutherford-Geiger-Marsden alpha-particle scattering experiment. Only those alphas that get close to the nucleus are scattered through significant angles. For a more quantitative approach more able students can measure the ratio of deflection angles for particles on different approach trajectories and compare this with Rutherfords scattering formula. The Rutherford scattering formula for the probability per unit area for alpha particles scattering into a ring around the angle θ called Nθ is. Rutherford Scattering - PhET Interactive Simulations.
 Source: pinterest.com
Source: pinterest.com
Two of his students Hans Geiger and Ernest Marsden an undergraduate set out to measure the number of alpha particles scattered out of a collimated beam upon hitting a thin metal foil. For a more quantitative approach more able students can measure the ratio of deflection angles for particles on different approach trajectories and compare this with Rutherfords scattering formula. The blue circle represents a heavy nucleus. The tutorial simulates diffraction of alpha particles helium nuclei containing two positive charges by a thin foil made of gold metal. Atom consists of central massive nucleus in which all the positive charge and most of the mass are concentrated.
 Source: youtube.com
Source: youtube.com
Opposite the gold foil is a zinc sulfide screen that emits a flash of light when struck by an alpha particle. Related videos -1how electric car works httpsyoutubegFHwWjdrp642how turbojet engine workshttpsyoutubeR_EmUgi77e03how radial engine works. A steady rain of alpha particles red circles comes from a source on the left. Rutherford Scattering Interactive Animation This animation allows you to simulate the Rutherford-Geiger-Marsden alpha-particle scattering experiment. Two of his students Hans Geiger and Ernest Marsden an undergraduate set out to measure the number of alpha particles scattered out of a collimated beam upon hitting a thin metal foil.
 Source: pinterest.com
Source: pinterest.com
That was Rutherford Atomic Model and obviously discovery of NUCLEUS 6 7. The blue circle represents a heavy nucleus. The Rutherford scattering formula for the probability per unit area for alpha particles scattering into a ring around the angle θ called Nθ is. Rutherford directed the GeigerMarsden experiment in 1909 which suggested upon Rutherfords 1911 analysis that J. Rutherfords Nuclear Atom ExperimentIn 1910 Rutherford and his coworkers were studying the angles at which alpha particles were scattered as they passed thr.
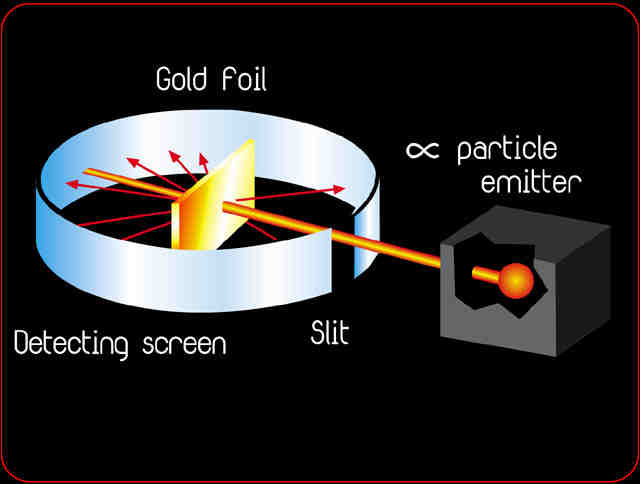 Source: kentchemistry.com
Source: kentchemistry.com
In 1911 Rutherford discovered the nucleus by analysing the data of Geiger and Marsden on the scattering of α-particles against a very thin foil of gold. A steady rain of alpha particles red circles comes from a source on the left. Only those alphas that get close to the nucleus are scattered through significant angles. Rutherford Scattering Let us start from the one of the first steps which was done towards understanding the deepest structure of matter. Ernest Rutherford in 1911 with his postulates concerning the scattering of alpha particles by atoms.
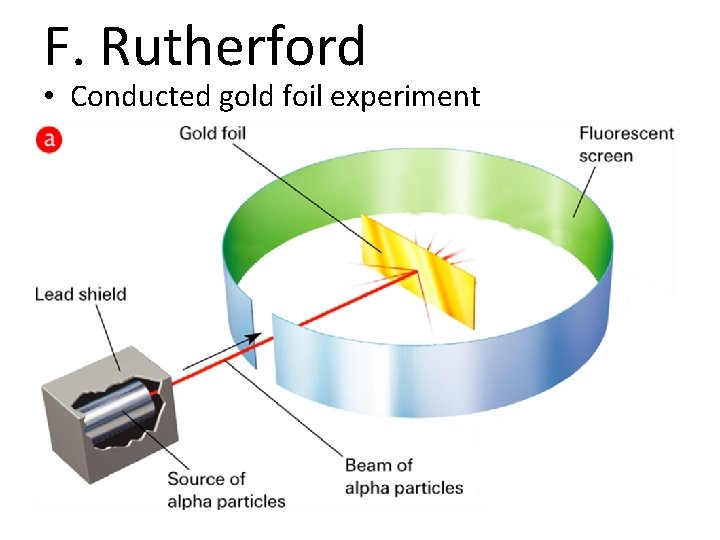 Source: slidetodoc.com
Source: slidetodoc.com
Rutherford Scattering Interactive Animation This animation allows you to simulate the Rutherford-Geiger-Marsden alpha-particle scattering experiment. Related videos -1how electric car works httpsyoutubegFHwWjdrp642how turbojet engine workshttpsyoutubeR_EmUgi77e03how radial engine works. For a more quantitative approach more able students can measure the ratio of deflection angles for particles on different approach trajectories and compare this with Rutherfords scattering formula. Rutherford Scattering - PhET Interactive Simulations. Rutherford Scattering - Atomic Nuclei Atomic Structure Quantum Mechanics - PhET Interactive Simulations.
 Source: mozaweb.com
Source: mozaweb.com
Related videos -1how electric car works httpsyoutubegFHwWjdrp642how turbojet engine workshttpsyoutubeR_EmUgi77e03how radial engine works. Ernest Rutherford in 1911 with his postulates concerning the scattering of alpha particles by atoms. A steady rain of alpha particles red circles comes from a source on the left. Rutherford Streuung - PhET Interactive Simulations. A cloud of negatively charged electrons surrounds this nucleus.
 Source: dreamstime.com
Source: dreamstime.com
3D animation of an atom incorporating the Rutherford model The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. The Rutherford scattering formula for the probability per unit area for alpha particles scattering into a ring around the angle θ called Nθ is. Just a little testFollow me on twitter andreabello00. Collected from the entire web and summarized to include only the most important parts of it. Rutherfords Nuclear Atom ExperimentIn 1910 Rutherford and his coworkers were studying the angles at which alpha particles were scattered as they passed thr.
 Source: prezi.com
Source: prezi.com
The blue circle represents a heavy nucleus. Rutherford Streuung - PhET Interactive Simulations. Wie hat RUTHERFORD die Struktur des Atoms herauszufinden ohne es tatsächlich zu sehen. Opposite the gold foil is a zinc sulfide screen that emits a flash of light when struck by an alpha particle. Atom consists of central massive nucleus in which all the positive charge and most of the mass are concentrated.
 Source: pinterest.com
Source: pinterest.com
In 1911 Rutherford discovered the nucleus by analysing the data of Geiger and Marsden on the scattering of α-particles against a very thin foil of gold. Rutherford model was accepted because he proved scattering of particles with mathematical formula. Nθ nt4r 2 zZ2K 2 e 2 4πε 0 2 1sin 4 θ2 48. A steady rain of alpha particles red circles comes from a source on the left. Thomson s plum pudding model of the atom was incorrect.
 Source: pngegg.com
Source: pngegg.com
Can be used as content for research and analysis. Atomic electrons would have negligible effect on the trajectories of the alpha particles. The tutorial simulates diffraction of alpha particles helium nuclei containing two positive charges by a thin foil made of gold metal. Can be used as content for research and analysis. Nθ nt4r 2 zZ2K 2 e 2 4πε 0 2 1sin 4 θ2 48.
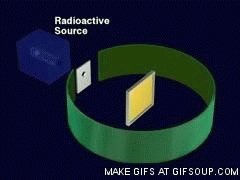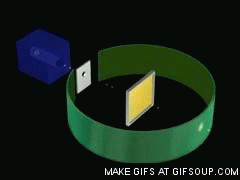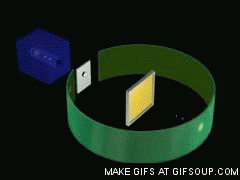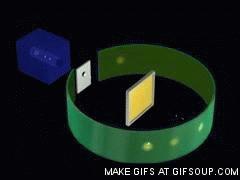
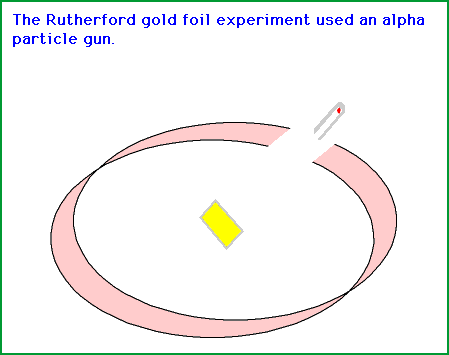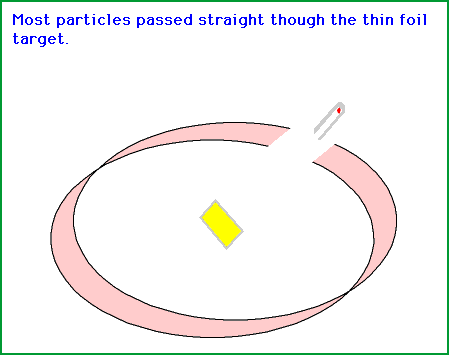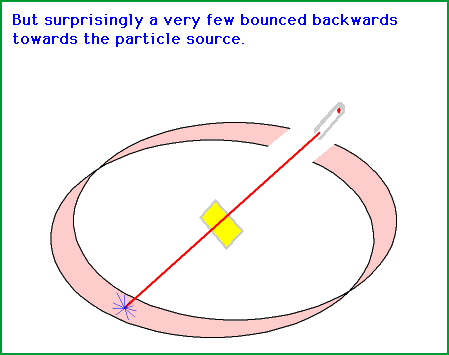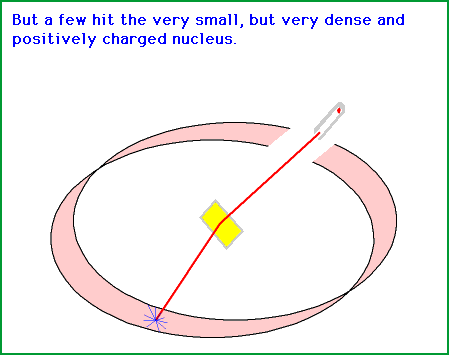 Source: gifer.com
Source: gifer.com
3D animation of an atom incorporating the Rutherford model The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. For a more quantitative approach more able students can measure the ratio of deflection angles for particles on different approach trajectories and compare this with Rutherfords scattering formula. In 1911 Rutherford discovered the nucleus by analysing the data of Geiger and Marsden on the scattering of α-particles against a very thin foil of gold. Ernest Rutherford in 1911 with his postulates concerning the scattering of alpha particles by atoms. Rutherfords Nuclear Atom ExperimentIn 1910 Rutherford and his coworkers were studying the angles at which alpha particles were scattered as they passed thr.
 Source: pt.dreamstime.com
Source: pt.dreamstime.com
The blue circle represents a heavy nucleus. Thomson s plum pudding model of the atom was incorrect. Opposite the gold foil is a zinc sulfide screen that emits a flash of light when struck by an alpha particle. 3D animation of an atom incorporating the Rutherford model The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. In 1911 Rutherford discovered the nucleus by analysing the data of Geiger and Marsden on the scattering of α-particles against a very thin foil of gold.
 Source: gifer.com
Source: gifer.com
For a more quantitative approach more able students can measure the ratio of deflection angles for particles on different approach trajectories and compare this with Rutherfords scattering formula. Atomic electrons would have negligible effect on the trajectories of the alpha particles. Only those alphas that get close to the nucleus are scattered through significant angles. Rutherford Scattering - PhET Interactive Simulations. Rutherford Scattering Let us start from the one of the first steps which was done towards understanding the deepest structure of matter.
 Source: co.pinterest.com
Source: co.pinterest.com
Rutherford Scattering Let us start from the one of the first steps which was done towards understanding the deepest structure of matter. Nθ nt4r 2 zZ2K 2 e 2 4πε 0 2 1sin 4 θ2 48. Atom consists of central massive nucleus in which all the positive charge and most of the mass are concentrated. 3D animation of an atom incorporating the Rutherford model The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. Can be used as content for research and analysis.
 Source: slideplayer.com
Source: slideplayer.com
The tutorial simulates diffraction of alpha particles helium nuclei containing two positive charges by a thin foil made of gold metal. Wie hat RUTHERFORD die Struktur des Atoms herauszufinden ohne es tatsächlich zu sehen. Thomson s plum pudding model of the atom was incorrect. Rutherford Scattering Let us start from the one of the first steps which was done towards understanding the deepest structure of matter. Rutherford Scattering - Atomic Nuclei Atomic Structure Quantum Mechanics - PhET Interactive Simulations.
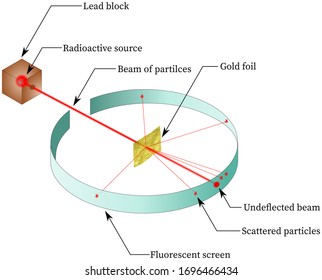 Source: shutterstock.com
Source: shutterstock.com
3D animation of an atom incorporating the Rutherford model The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. Rutherford model was accepted because he proved scattering of particles with mathematical formula. Nθ nt4r 2 zZ2K 2 e 2 4πε 0 2 1sin 4 θ2 48. The tutorial simulates diffraction of alpha particles helium nuclei containing two positive charges by a thin foil made of gold metal. A steady rain of alpha particles red circles comes from a source on the left.
 Source: youtube.com
Source: youtube.com
3D animation of an atom incorporating the Rutherford model The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. Thomson s plum pudding model of the atom was incorrect. The Rutherford scattering formula for the probability per unit area for alpha particles scattering into a ring around the angle θ called Nθ is. For a more quantitative approach more able students can measure the ratio of deflection angles for particles on different approach trajectories and compare this with Rutherfords scattering formula. Only those alphas that get close to the nucleus are scattered through significant angles.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title rutherford scattering animation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





