Your Production of vaccines in animal cells ppt images are ready in this website. Production of vaccines in animal cells ppt are a topic that is being searched for and liked by netizens today. You can Find and Download the Production of vaccines in animal cells ppt files here. Get all royalty-free photos and vectors.
If you’re searching for production of vaccines in animal cells ppt images information related to the production of vaccines in animal cells ppt keyword, you have pay a visit to the right blog. Our site always gives you suggestions for seeking the highest quality video and image content, please kindly hunt and find more informative video content and images that match your interests.
Production Of Vaccines In Animal Cells Ppt. The conjugate retrieved by this mechanism is then processed and the peptides derived from the carrier proteins are loaded into the MHC cavity and presented to the T cells. Inactivated vaccines elicit an immune response but the response often is less complete than with attenuated vaccines. The cells are first grown in a small volume of culture medium. Another way to produce a highly effective living vaccine is to insert the genes that encode protective antigens into an avirulent vector organism.
 Animal Cell Culture Techniques From slideshare.net
Animal Cell Culture Techniques From slideshare.net
For example To produce the Sabin polio vaccine attenuation was only achieved with high inocula and rapid passage in primary monkey kidney cells. Inactivated vaccines are produced by killing the disease -causing. Among the biological products whose production involves animal cell cultivation techniques are viral vaccines human and veterinary monoclonal antibodies cell surface antigens. They provide the help necessary to start the GC reaction that leads to affinity maturation proliferation and the production of plasma cells which produce polysaccharide-specific antibodies and. Conference of Catholic Bishops goes further and. 9 The requirements and procedures described here are intended to be general in nature and to be 10 consistent with published standards that are generally available for guidance in the production of 11 veterinary vaccines.
The Catholic Church and the Southern Baptist Ethics Religious Liberty Commission have both stated that receiving a COVID-19 vaccine that required fetal cell lines for production or manufacture is morally acceptable.
The transgenic plant produces large amounts of antigen that can be used as a vaccine. Proteins produced naturally by the B-lymphocytes that bind to specific antigens. Cells begin to replicate and produce antibody rapidly to reestablish protection. Another way to produce active immunity is by vaccination. Antibodies may be divided into two main types monoclonal and polyclonal antibodies based on. They may produce a mild or subclinical form of the disease.
 Source: slideplayer.com
Source: slideplayer.com
For the 2020-2021 season the viruses provided to the manufacturer to be grown in cell culture are cell-derived rather than egg-derived. The cells are first grown in a small volume of culture medium. The cell-culture vaccine process is suitable for large-scale manufacture and the process parameters can be ramped up and run routinely and cost effectively. Vaccines contain antigens that stimulate the immune system to produce an immune response that is often similar to that produced by the natural infection. Conference of Catholic Bishops goes further and.
 Source: slideserve.com
Source: slideserve.com
The transgenic plant produces large amounts of antigen that can be used as a vaccine. For the 2020-2021 season the viruses provided to the manufacturer to be grown in cell culture are cell-derived rather than egg-derived. MRNA vaccines have the potential for rapid inexpensive and scalable manufacturing mainly owing to the high yields of in vitro transcription reactions. They may produce a mild or subclinical form of the disease. As the cells grow and multiply they are transferred to successively larger containers.
 Source: irp.nih.gov
Source: irp.nih.gov
Another way to produce active immunity is by vaccination. Inactivated vaccines are those that contain organisms that have been killed or inactivated with heat or chemicals. As the cells grow and multiply they are transferred to successively larger containers. Inactivated vaccines elicit an immune response but the response often is less complete than with attenuated vaccines. Owing to inherent properties of the lipid envelope assembly of VLPs in insect cells for these viral vaccines is a different type of technical challenge to those produced viruses with multiple capsids.
 Source: slideshare.net
Source: slideshare.net
DNA vaccines are produced by engineering genes for protective antigens into bacterial plasmids circular pieces of DNA. As the cells grow and multiply they are transferred to successively larger containers. They provide the help necessary to start the GC reaction that leads to affinity maturation proliferation and the production of plasma cells which produce polysaccharide-specific antibodies and. The cell-based vaccine manufacturing process uses animal cells Madin-Darby Canine Kidney or MDCK cells as a host for the growing flu viruses instead of fertilized chicken eggs. 9 The requirements and procedures described here are intended to be general in nature and to be 10 consistent with published standards that are generally available for guidance in the production of 11 veterinary vaccines.
 Source: slideshare.net
Source: slideshare.net
The Catholic Church and the Southern Baptist Ethics Religious Liberty Commission have both stated that receiving a COVID-19 vaccine that required fetal cell lines for production or manufacture is morally acceptable. The conjugate retrieved by this mechanism is then processed and the peptides derived from the carrier proteins are loaded into the MHC cavity and presented to the T cells. These vaccines are created by deleting genes from the vector and replacing them with genes coding for antigens from the pathogen. Inactivated vaccines are produced by killing the disease -causing. Inactivated vaccines are those that contain organisms that have been killed or inactivated with heat or chemicals.
 Source: pinterest.com
Source: pinterest.com
Owing to inherent properties of the lipid envelope assembly of VLPs in insect cells for these viral vaccines is a different type of technical challenge to those produced viruses with multiple capsids. DNA vaccines are produced by engineering genes for protective antigens into bacterial plasmids circular pieces of DNA. The conjugate retrieved by this mechanism is then processed and the peptides derived from the carrier proteins are loaded into the MHC cavity and presented to the T cells. Conference of Catholic Bishops goes further and. MRNA vaccines have the potential for rapid inexpensive and scalable manufacturing mainly owing to the high yields of in vitro transcription reactions.
 Source: slideshare.net
Source: slideshare.net
MRNA vaccines have the potential for rapid inexpensive and scalable manufacturing mainly owing to the high yields of in vitro transcription reactions. Another way to produce active immunity is by vaccination. 1 For these targets production of VLPs is a challenging task because the synthesis and assembly of one or more recombinant proteins may be required. In other cases 8 vaccines are used in conjunction with national disease control or eradication programmes. Inactivated vaccines are produced by killing the disease -causing.
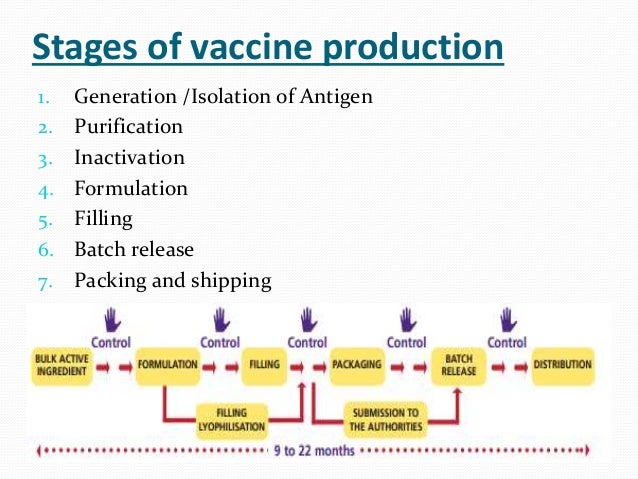 Source: slideshare.net
Source: slideshare.net
Inactivated vaccines elicit an immune response but the response often is less complete than with attenuated vaccines. Among the biological products whose production involves animal cell cultivation techniques are viral vaccines human and veterinary monoclonal antibodies cell surface antigens. Vaccines contain antigens that stimulate the immune system to produce an immune response that is often similar to that produced by the natural infection. Conference of Catholic Bishops goes further and. The cell-based vaccine manufacturing process uses animal cells Madin-Darby Canine Kidney or MDCK cells as a host for the growing flu viruses instead of fertilized chicken eggs.
 Source: slideshare.net
Source: slideshare.net
These vaccines are created by deleting genes from the vector and replacing them with genes coding for antigens from the pathogen. They may produce a mild or subclinical form of the disease. As the cells grow and multiply they are transferred to successively larger containers. Cells begin to replicate and produce antibody rapidly to reestablish protection. Inactivated vaccines are those that contain organisms that have been killed or inactivated with heat or chemicals.
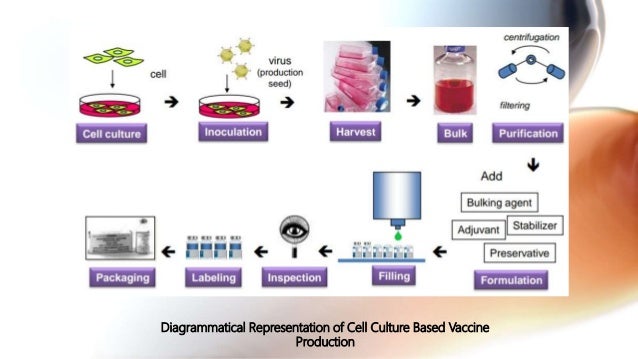 Source: slideshare.net
Source: slideshare.net
Fetal cell cultures specifically PERC6 in order to produce and manufacture the vaccine. Cell Culture Production In order to make cell culture-based influenza vaccines frozen preserved cells are taken from storage and grown in an incubator at 37C. They provide the help necessary to start the GC reaction that leads to affinity maturation proliferation and the production of plasma cells which produce polysaccharide-specific antibodies and. Inactivated vaccines are those that contain organisms that have been killed or inactivated with heat or chemicals. For example To produce the Sabin polio vaccine attenuation was only achieved with high inocula and rapid passage in primary monkey kidney cells.
 Source: slideplayer.com
Source: slideplayer.com
The cell-culture vaccine process is suitable for large-scale manufacture and the process parameters can be ramped up and run routinely and cost effectively. MRNA vaccines have the potential for rapid inexpensive and scalable manufacturing mainly owing to the high yields of in vitro transcription reactions. Inactivated vaccines are those that contain organisms that have been killed or inactivated with heat or chemicals. Using rDNA technology antibodies are also produced in other continuous cell lines. This is the case for VLPs of rotavirus which is an RNA virus with.
 Source: slideshare.net
Source: slideshare.net
Inactivated vaccines elicit an immune response but the response often is less complete than with attenuated vaccines. Vaccines contain antigens that stimulate the immune system to produce an immune response that is often similar to that produced by the natural infection. Described lessons from animal and insect derived vaccines. Proteins produced naturally by the B-lymphocytes that bind to specific antigens. Cell Culture Production In order to make cell culture-based influenza vaccines frozen preserved cells are taken from storage and grown in an incubator at 37C.
 Source: researchgate.net
Source: researchgate.net
Conference of Catholic Bishops goes further and. Using rDNA technology antibodies are also produced in other continuous cell lines. Therefore with conjugate vaccines T cells are engaged. The conjugate retrieved by this mechanism is then processed and the peptides derived from the carrier proteins are loaded into the MHC cavity and presented to the T cells. These vaccines are created by deleting genes from the vector and replacing them with genes coding for antigens from the pathogen.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Currently available vaccines and biologicals are derived from cells originated in humans MRC5 WI38 and others monkeys primary kidney chickens chick embryo fibroblast or rodents hybridomas Chinese hamster ovary primary hamster kidney. 1 For these targets production of VLPs is a challenging task because the synthesis and assembly of one or more recombinant proteins may be required. The cell-culture vaccine process is suitable for large-scale manufacture and the process parameters can be ramped up and run routinely and cost effectively. Cell cultures are required for viral vaccines since viruses can replicate only inside the living cells. Inactivated vaccines elicit an immune response but the response often is less complete than with attenuated vaccines.
 Source: br.pinterest.com
Source: br.pinterest.com
The cells are first grown in a small volume of culture medium. For the 2020-2021 season the viruses provided to the manufacturer to be grown in cell culture are cell-derived rather than egg-derived. Owing to inherent properties of the lipid envelope assembly of VLPs in insect cells for these viral vaccines is a different type of technical challenge to those produced viruses with multiple capsids. Attenuated vaccines include those for measles mumps polio the Sabin vaccine rubella and tuberculosis. DNA vaccines are produced by engineering genes for protective antigens into bacterial plasmids circular pieces of DNA.
 Source: slideshare.net
Source: slideshare.net
Fetal cell cultures specifically PERC6 in order to produce and manufacture the vaccine. Plant derived vaccines use genes from animal pathogens that can be genetically engineered into a plant. Cells begin to replicate and produce antibody rapidly to reestablish protection. Fetal cell cultures specifically PERC6 in order to produce and manufacture the vaccine. For the 2020-2021 season the viruses provided to the manufacturer to be grown in cell culture are cell-derived rather than egg-derived.
 Source: slideshare.net
Source: slideshare.net
The cell-based vaccine manufacturing process uses animal cells Madin-Darby Canine Kidney or MDCK cells as a host for the growing flu viruses instead of fertilized chicken eggs. They provide the help necessary to start the GC reaction that leads to affinity maturation proliferation and the production of plasma cells which produce polysaccharide-specific antibodies and. Therefore with conjugate vaccines T cells are engaged. Inactivated vaccines are produced by killing the disease -causing. Attenuated vaccines include those for measles mumps polio the Sabin vaccine rubella and tuberculosis.
 Source: pinterest.com
Source: pinterest.com
Another way to produce a highly effective living vaccine is to insert the genes that encode protective antigens into an avirulent vector organism. For the 2020-2021 season the viruses provided to the manufacturer to be grown in cell culture are cell-derived rather than egg-derived. Inactivated vaccines elicit an immune response but the response often is less complete than with attenuated vaccines. Proteins produced naturally by the B-lymphocytes that bind to specific antigens. In other cases 8 vaccines are used in conjunction with national disease control or eradication programmes.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title production of vaccines in animal cells ppt by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





